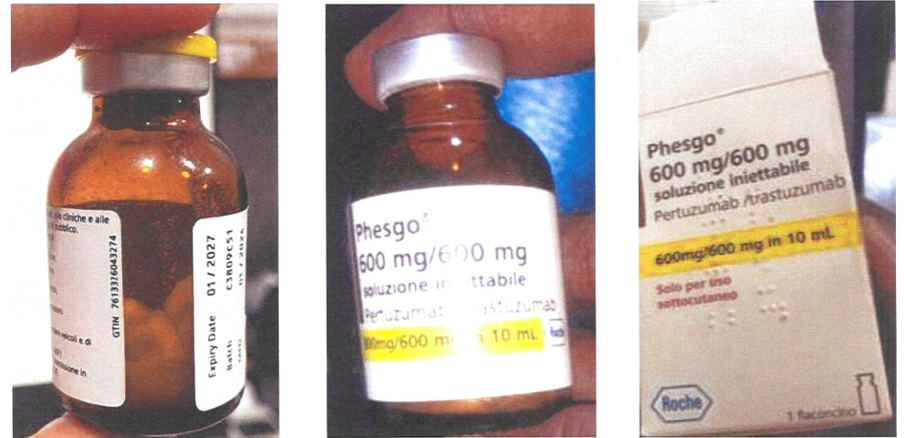
The National Agency for Food and Drugs Administration and Control (NAFDAC) has issued a warning about a suspected counterfeit of Phesgo® 600mg/10ml with batch number C3809C51.
According to NAFDAC, Roche, the Marketing Authorisation Holder, received a complaint from a pharmacist regarding the counterfeit batch.
Although no physical sample was returned to Roche, photographs revealed significant differences from genuine Phesgo® products.
Issues included mismatched batch numbers, incorrect tamper evidence labels, and discrepancies in vial dimensions and labeling.
“The counterfeit product, which is a solid instead of the genuine liquid, resembles other counterfeit cases reported in Libya. The similarity includes batch numbers and Bollino labels.
“NAFDAC has instructed its regional coordinators to increase surveillance and remove counterfeit products. Importers, distributors, and healthcare providers are advised to verify product authenticity and report any suspicions to NAFDAC.
“Healthcare professionals and consumers can report suspected counterfeits or adverse effects to NAFDAC via phone, email, or online platforms. This alert will also be shared with the WHO Global Surveillance and Monitoring System.”